WASHINGTON — U.S. researchers will quickly take a look at whether or not livers from a gene-edited pig might deal with folks with sudden liver failure — by quickly filtering their blood so their very own organ can relaxation and perhaps heal.
The primary-of-its-kind scientific trial has been cleared by the Meals and Drug Administration, in response to pig producer eGenesis, which introduced the step Tuesday with its companion OrganOx.
An estimated 35,000 folks within the U.S. are hospitalized every year when their liver out of the blue fails. There are few therapy choices and dying charges as excessive as 50%. Many do not qualify for a liver transplant or cannot get a match in time.
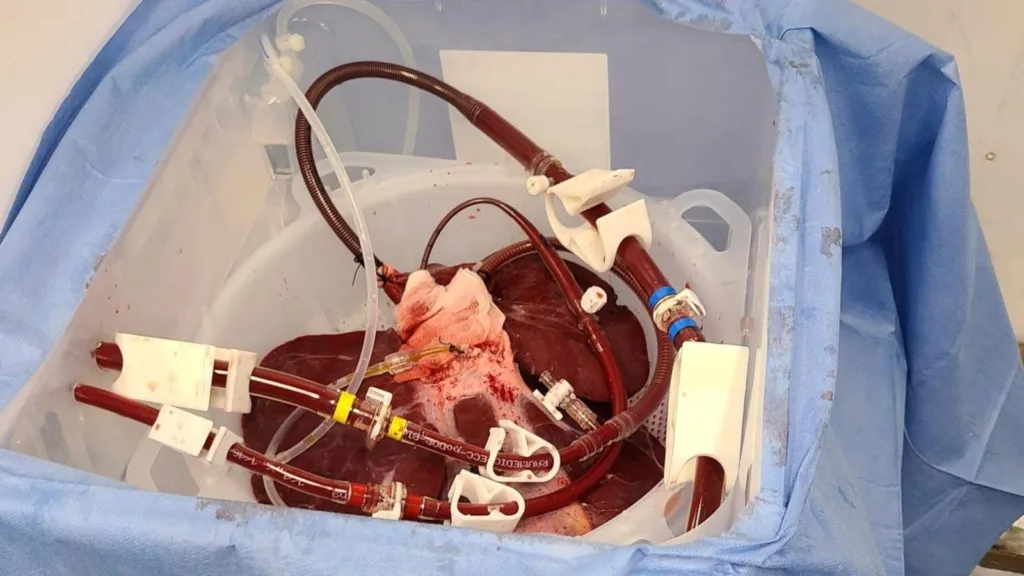
The brand new examine, which is predicted to get underway later this spring, is a twist on the search for animal-to-human organ transplants. Researchers will not transplant the pig liver however as a substitute will connect it externally to review contributors.
The liver is the one organ that may regenerate, however the query is whether or not having the pig’s liver filter the affected person’s blood for a number of days might give it that likelihood.
In experiments with 4 deceased our bodies, that “bridge” try confirmed the pig liver might assist some features of a human liver for 2 or three days, stated Mike Curtis, CEO of Massachusetts-based eGenesis, which genetically modifies pigs so their organs are extra humanlike.
The trial will enroll as much as 20 sufferers in intensive-care models who do not qualify a liver transplant, he stated. A tool made by Britain’s OrganOx, at the moment used to protect donated human livers, will pump contributors’ blood by means of the pig liver.
It’s the newest step in makes an attempt to make use of gene-edited pig organs to avoid wasting human lives. Pig kidneys from eGenesis and one other pig producer, United Therapeutics, are being utilized in experimental transplants.
___
The Related Press Well being and Science Division receives assist from the Howard Hughes Medical Institute’s Science and Instructional Media Group and the Robert Wooden Johnson Basis. The AP is solely accountable for all content material.