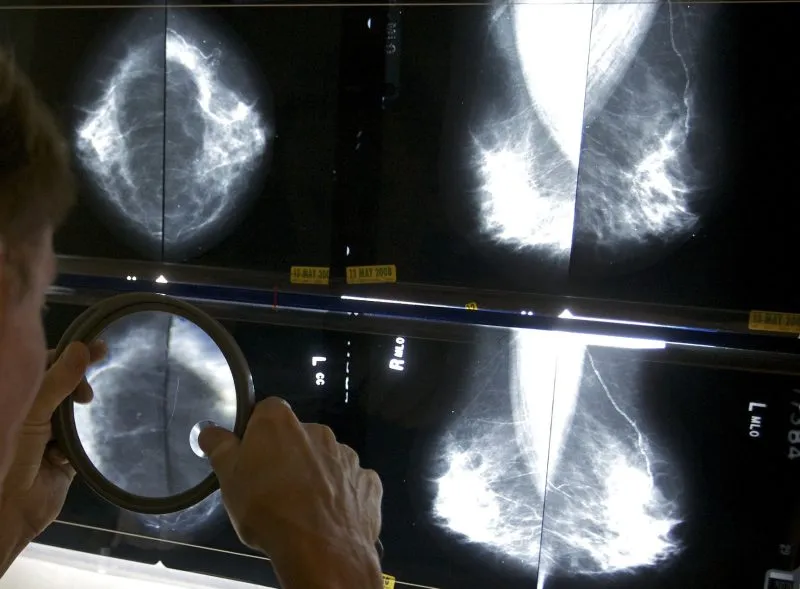
(NewsNation) — Mammogram providers are now federally required to tell their patients about their breast density, which not only increases the risk of cancer but makes it harder to detect.
The new U.S. Food and Drug Administration standards for the procedure were first released in March 2023, with an implementation deadline of Sept. 9, 2024.
According to the FDA, around half of women 40 or older in the United States have dense breast tissue.
“Dense breast tissue can make cancers more difficult to detect on a mammogram. Additionally, dense breasts have been identified as a risk factor for developing breast cancer,” the FDA said in a statement.
About 1 in 8 women will get breast cancer in her life, according to the Centers for Disease Control and Prevention. It’s the most common cancer in the nation, with around 310,000 new cases detected in men and women yearly.
Having information about breast density could help patients handle their health better as “an important part of a comprehensive breast health strategy,” the FDA explained.
Those with dense breasts might require more than just a mammogram — like additional MRIs or ultrasounds — to assess cancer risks.
Denser breasts are typically found on younger people with lower body weights. Pregnant, breastfeeding and hormone replacement therapy patients also tend to have denser breasts.